Danni Zheng 1 2 3 4 5 6, Lin Zeng 7, Rui Yang 1 2 3 4 5 6, Ying Lian 1 2 3 4 5 6, Yi-Min Zhu 8, Xiaoyan Liang 9, Li Tang 10, Huichun Wang 11, Yunxia Cao 12, Guimin Hao 13, Jianqiao Liu 14, Junli Zhao 15, Rui Wang 16 17, Ben Willem Mol 17, Rong Li 1 2 3 4 5 6, He-Feng Huang 18, Jie Qiao 19 2 3 4 5 6
Affiliations Expand
- PMID: 31575574
- PMCID: PMC6773417
- DOI: 10.1136/bmjopen-2019-030366
Abstract
Introduction: Intracytoplasmic sperm injection (ICSI), originally introduced as add-on to in vitro fertilisation (IVF) for couples with severe male infertility, is in current clinical practice also used in couples with mild male or even unexplained infertility. However, ICSI has involved unresolved concerns regarding the selection and damage to gametes and the health conditions of the offspring, and it is also labour intensive and therefore more expensive than conventional IVF. High-quality well-powered randomised clinical trials (RCTs) comparing ICSI and IVF are lacking.
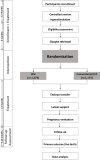
Methods and analysis: We propose a multicentre, open-label RCT in 10 reproductive medical centres across China. We will study couples with non-severe male infertility (defined as a semen concentrate 5-15×106/mL or sperm with a progressive motility 10%-32%) scheduled for their first or second ICSI or IVF cycle, as low fertility rate after fertilisation are more frequent in this population, which could lead to controversy about ICSI or conventional IVF for fertilisation. On the day of oocyte retrieval, eligible participants are after informed consent be randomised to undergo either ICSI or conventional IVF in a 1:1 treatment ratio. Other standard assisted reproductive treatments are similar and parallel between two groups. Our primary outcome is ongoing pregnancy leading to live birth after the first cycle with embryo transfer. To demonstrate or refute a difference of 7% between ICSI and conventional IVF, we need to include 2346 women (1173 in each intervention arm). In addition, we will follow-up neonatal outcomes after delivery to identify the influence of ICSI on offspring.
Ethics and dissemination: Ethical approval was obtained from Peking University Third Hospital medical science research ethics committee. The findings will be disseminated to the public through conference presentations and peer-reviewed scientific journals.
Trial registration number: ClinicalTrials.gov registry (NCT03298633).
Keywords: assisted reproductive technology; in vitro fertilisation; intracytoplasmic sperm injection; non-severe male infertility.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
- Intracytoplasmic sperm injection versus conventional in-vitro fertilisation for couples with infertility with non-severe male factor: a multicentre, open-label, randomised controlled trial.Wang Y, Li R, Yang R, Zheng D, Zeng L, Lian Y, Zhu Y, Zhao J, Liang X, Li W, Liu J, Tang L, Cao Y, Hao G, Wang H, Zhang H, Wang R, Mol BW, Huang H, Qiao J.Lancet. 2024 Mar 9;403(10430):924-934. doi: 10.1016/S0140-6736(23)02416-9. Epub 2024 Feb 5.PMID: 38330980 Clinical Trial.
- In vitro fertilisation (IVF) versus intracytoplasmic sperm injection (ICSI) in patients without severe male factor infertility: study protocol for the randomised, controlled, multicentre trial INVICSI.Berntsen S, Nøhr B, Grøndahl ML, Petersen MR, Andersen LF, Englund AL, Knudsen UB, Prætorius L, Zedeler A, Nielsen HS, Pinborg A, Freiesleben NC.BMJ Open. 2021 Jun 24;11(6):e051058. doi: 10.1136/bmjopen-2021-051058.PMID: 34168037 Free PMC article.
- Preimplantation genetic testing for aneuploidy in severe male factor infertility: protocol for a multicenter randomised controlled trial.Lin XH, Guo MX, Wu DD, Lu Y, Zhang JL, Zhou CL, Jin L, Wang L, Zhang C, Xu CM, Chen SC, Zhang SY, Sun XX, Wu YT, Sun Y, Huang HF.BMJ Open. 2022 Jul 13;12(7):e063030. doi: 10.1136/bmjopen-2022-063030.PMID: 35831058 Free PMC article.
- Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction.Teixeira DM, Hadyme Miyague A, Barbosa MA, Navarro PA, Raine-Fenning N, Nastri CO, Martins WP.Cochrane Database Syst Rev. 2020 Feb 21;2(2):CD010167. doi: 10.1002/14651858.CD010167.pub3.PMID: 32083321 Free PMC article.
- [Twenty years of in vitro fertilization: realization and questions for the future].van Steirteghem A.Verh K Acad Geneeskd Belg. 2001;63(3):193-240; discussion 240-1.PMID: 11499344 Review. Dutch.
Cited by
- The role of clinical trials in advancing reproductive medicine: a comprehensive overview.Tian T, Qiao J.Med Rev (2021). 2023 Dec 5;3(5):363-365. doi: 10.1515/mr-2023-0053. eCollection 2023 Oct.PMID: 38283257 Free PMC article. No abstract available.
- Sexual and Reproductive Health Among Men With Cystic Fibrosis.Campbell K, Zarli M, Schuppe K, Wong R, Rahman F, Ramasamy R.Urology. 2023 Sep;179:9-15. doi: 10.1016/j.urology.2023.06.017. Epub 2023 Jun 26.PMID: 37380131 Free PMC article. Review.
- Alkali and alkaline earth elements in follicular fluid and the likelihood of diminished ovarian reserve in reproductive-aged women: a case‒control study.Tian T, Li Q, Liu F, Jiang H, Yang R, Zhao Y, Kong F, Wang Y, Long X, Qiao J.J Ovarian Res. 2024 May 18;17(1):108. doi: 10.1186/s13048-024-01414-3.PMID: 38762521 Free PMC article.
- Effect of Paternal Body Mass Index on Cumulative Live Birth Rates: Retrospective Analysis of 3048 Embryo Transfers in Couples Using Autologous Gametes.Mossetti L, Hervás-Herrero I, Gil-Juliá M, Navarro Gomez-Lechon A, Pacheco-Rendón RM, Rivera-Egea R, Garrido-Puchalt N.Cells. 2024 Nov 6;13(22):1836. doi: 10.3390/cells13221836.PMID: 39594585 Free PMC article.
- Glucocorticoid therapy in assisted reproduction.Kim YJ.Clin Exp Reprod Med. 2021 Dec;48(4):295-302. doi: 10.5653/cerm.2021.04819. Epub 2021 Nov 30.PMID: 34875736 Free PMC article.
References
- Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab 2011;25:271–85. 10.1016/j.beem.2010.08.006 – DOI – PubMed
- Hull MG, Glazener CM, Kelly NJ, et al. . Population study of causes, treatment, and outcome of infertility. BMJ 1985;291:1693–7. 10.1136/bmj.291.6510.1693 – DOI – PMC – PubMed
- Crosignani PG, Walters DE. Clinical pregnancy and male subfertility; the ESHRE multicentre trial on the treatment of male subfertility. European Society of human reproduction and embryology. Hum Reprod 1994;9:1112–8. 10.1093/oxfordjournals.humrep.a138642 – DOI – PubMed
Show all 40 references
Publication types
MeSH terms
- China
- Female
- Fertilization in Vitro* / methods
- Humans
- Infertility, Male*
- Male
- Multicenter Studies as Topic
- Randomized Controlled Trials as Topic
- Sperm Injections, Intracytoplasmic* / methods
Associated data
Related information
LinkOut – more resources
- Full Text Sources
- Medical